Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect
€ 9.00 · 4.9 (277) · En stock
Por un escritor de hombre misterioso


Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect

Misfolding and Aggregation of SOD1 Wang et al.'s correlation of mutant

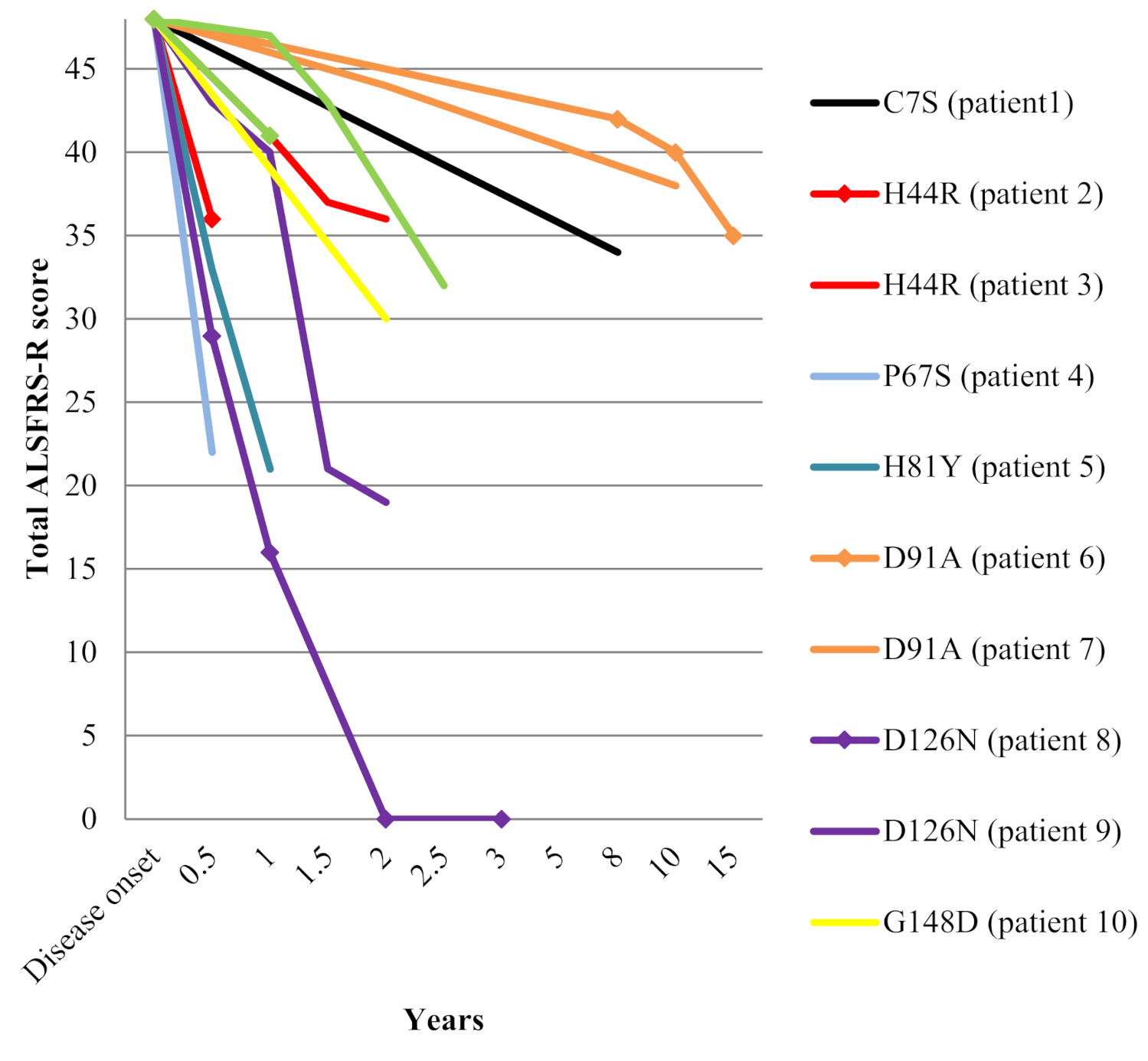
Molecular dynamics analysis of superoxide dismutase 1 mutations suggests decoupling between mechanisms underlying ALS onset and progression - Computational and Structural Biotechnology Journal
IJMS, Free Full-Text
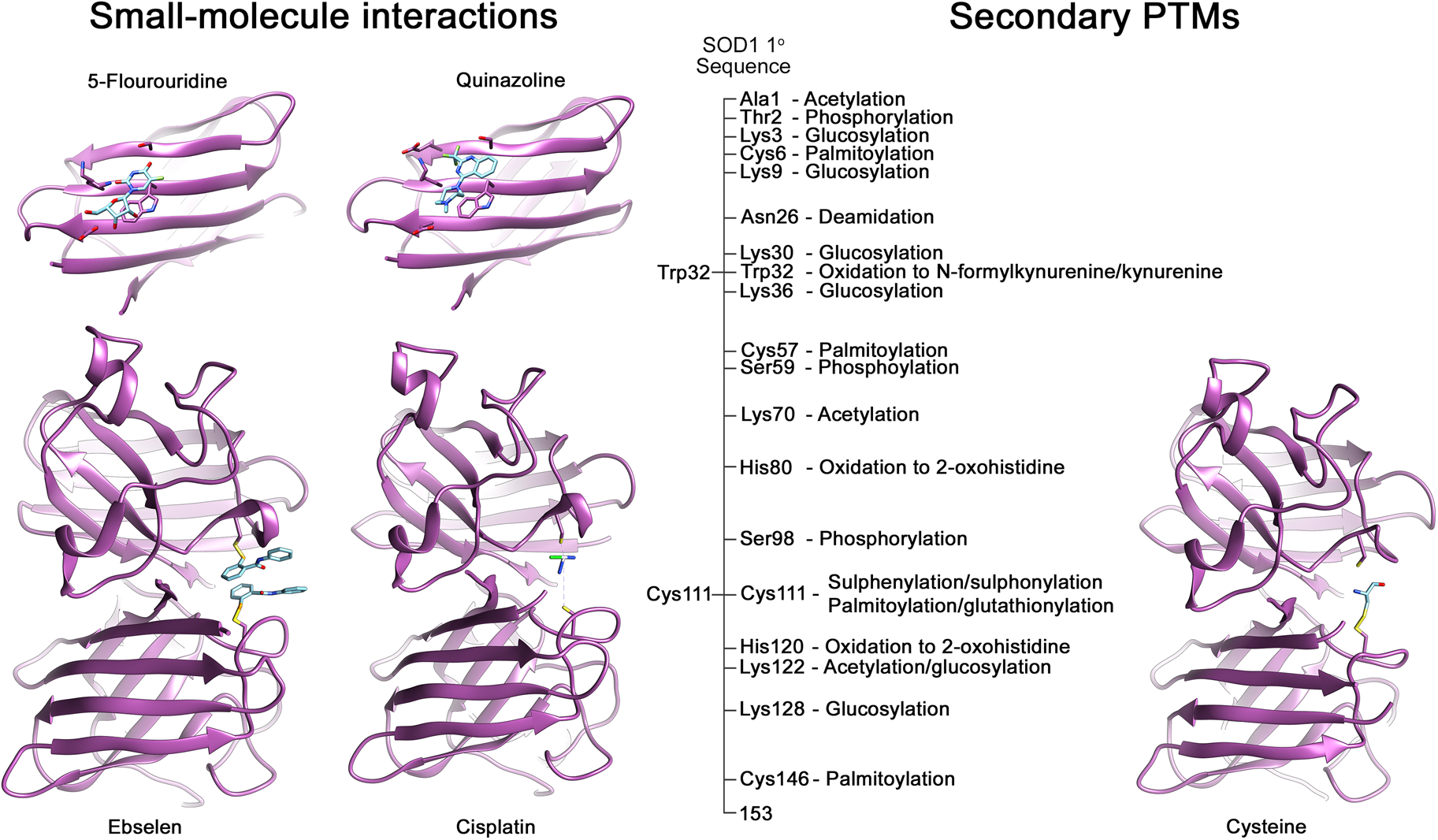
The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis, Quarterly Reviews of Biophysics

Molecular Mechanisms of Amyotrophic Lateral Sclerosis - ScienceDirect

Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect
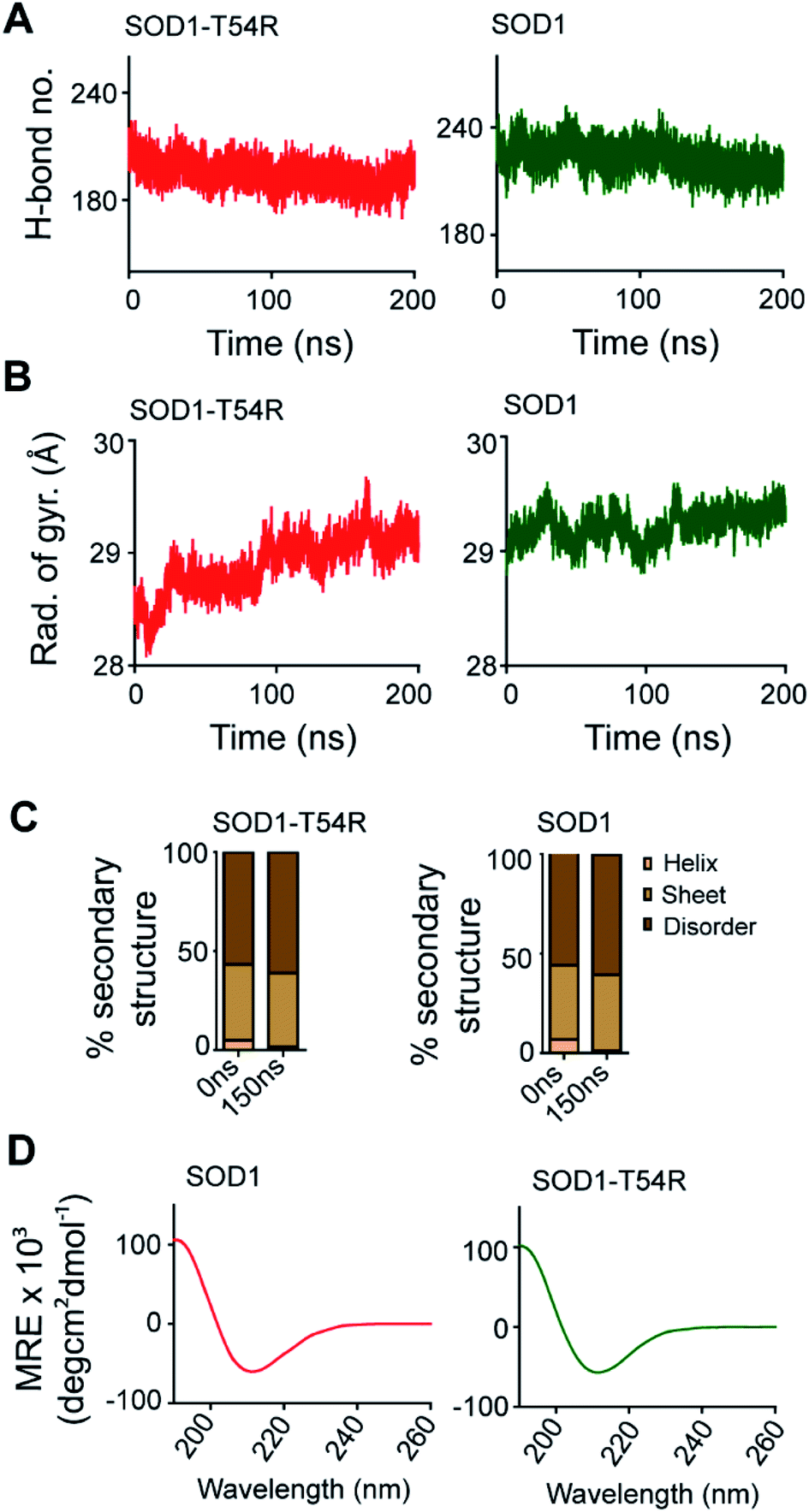
T54R mutation destabilizes the dimer of superoxide dismutase 1 T54R by inducing steric clashes at the dimer interface - RSC Advances (RSC Publishing) DOI:10.1039/C9RA09870D
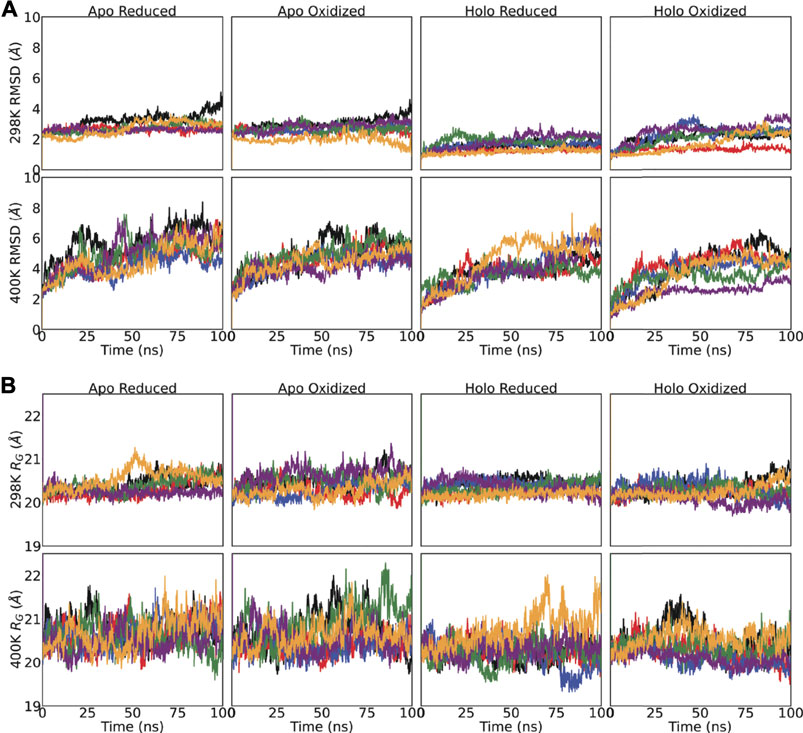
Frontiers Bridging the Bridging Imidazolate in the Bimetallic Center of the Cu/Zn SOD1 and ALS

A , WT SOD1 structure showing the position of the C57-C146 intrasubunit

Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect

Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect